To support pandemic response and preparedness efforts, today, Vitalant became the first U.S. blood services provider to release COVID-19 antibody-positive rates of more than 250,000 blood donors, June through July 2020. Those with a positive diagnostic or antibody test result are encouraged to consider registering to become a convalescent plasma donor and potentially help patients fight the disease.
COVID-19 Antibody Testing
On June 1, 2020, Vitalant was the first national blood services provider to begin testing all blood donations for antibodies to SARS-CoV-2, the coronavirus that causes COVID-19. The testing helps to identify donors who could help COVID-19 patients by becoming future convalescent plasma donors.
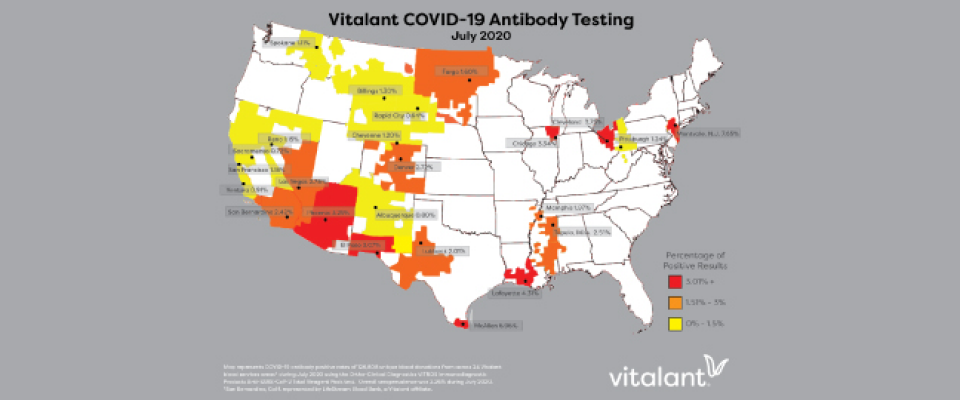
More than 250,000 total donors were tested, with an overall positive antibody rate of 2.26% in July, up from 1.37% in June. Vitalant’s blood service area based out of Montvale, New Jersey had the highest prevalence in July with 7.65% while Rapid City, South Dakota had the lowest in July at 0.64%.
“We are pleased to share our data to assist ongoing response and preparedness efforts,” said Ralph Vassallo, M.D., chief medical and scientific officer at Vitalant. “We will continue antibody testing all blood donations for the foreseeable future to help identify convalescent plasma donors and meet the emergent need.”
Donors are not charged for the test and costs are subsidized, in part, by The Blood Center Foundation of the Inland Northwest, Blood Science Foundation and The Bonfils Blood Center Donor Advised Fund.
Convalescent Plasma Donations
Plasma is the liquid, antibody-rich part of blood. Collected from recovered patients, this convalescent plasma has been used to treat the sick for more than 100 years and may give patients an immune system boost to fight disease. In April 2020, Vitalant began collecting and distributing convalescent plasma from those recovered from COVID-19.
“Although there are ongoing discussions regarding the Food and Drug Administration’s Emergency Use Authorization for convalescent plasma, we believe its risk-benefit profile is favorable, and plasma may be effective in hospitalized patients,” Vassallo continued. “Vitalant supports evidence-based decision-making and will continue to advocate for randomized trials to prove its efficacy and identify appropriate recipients. In the meantime, our focus remains on helping to save lives."
“Convalescent plasma donations are being distributed at a rapid clip – and we need to collect for immediate needs, as well as to be prepared for a second wave of infections,” said Cliff Numark, chief of marketing and senior vice president of donor services. “Vitalant’s goal is to double donations in the coming weeks and months and that is why we ask those who have recovered to help current patients in need."
Blood donors with a COVID-19 positive diagnostic or antibody test result can register to give convalescent plasma by calling 866-CV-PLSMA (866-287-5762). Donations can be made every 28 days or more frequently—up to every seven days—with Vitalant medical director approval.
Giving Blood is an Essential Activity
Every two seconds, someone in the U.S. needs blood. Blood and platelet donations are needed throughout this pandemic to help trauma victims, cancer patients and others with serious medical conditions.
The U.S. Surgeon General and Federal Emergency Management Agency (FEMA) designated blood donation as an essential activity, encouraging healthy and eligible donors to continue to donate during the COVID-19 pandemic. From coast to coast, all Vitalant donation centers and blood drives continue to deploy strict precautionary measures to ensure the safety of donors, patients and staff, including:
- Taking donors' temperatures upon check-in (staff self-monitor their temperatures)
- Requiring face masks or cloth-based face coverings (donors and staff)
- Disinfecting donor-touched and other high-touch areas often and after every donation
- Ensuring social distancing to keep donors and staff safe